Quality Inspection with SAP S4/HANA
„Quality inspections are the backbone of successful quality management. “
Standardization not only offers advantages in the context of SAP quality planning, but also increases the acceptance, satisfaction, and motivation of the workforce, reduces IT costs, improves the validity of collected inspection data, and creates transparency along the entire manufacturing process. In the context of production inspection, you ensure the maintenance and increase of customer satisfaction.
Quality inspection is one of the most important processes in the context of quality control and quality assurance. In this process, a material or product is tested based on inspection specifications with regard to the specified requirement and the results are documented for further use.
SAP Quality Inspection is the backbone of the entire QM process in SAP and links to almost every process area, such as supplier release, first article inspection, or quality assurance for goods issue.
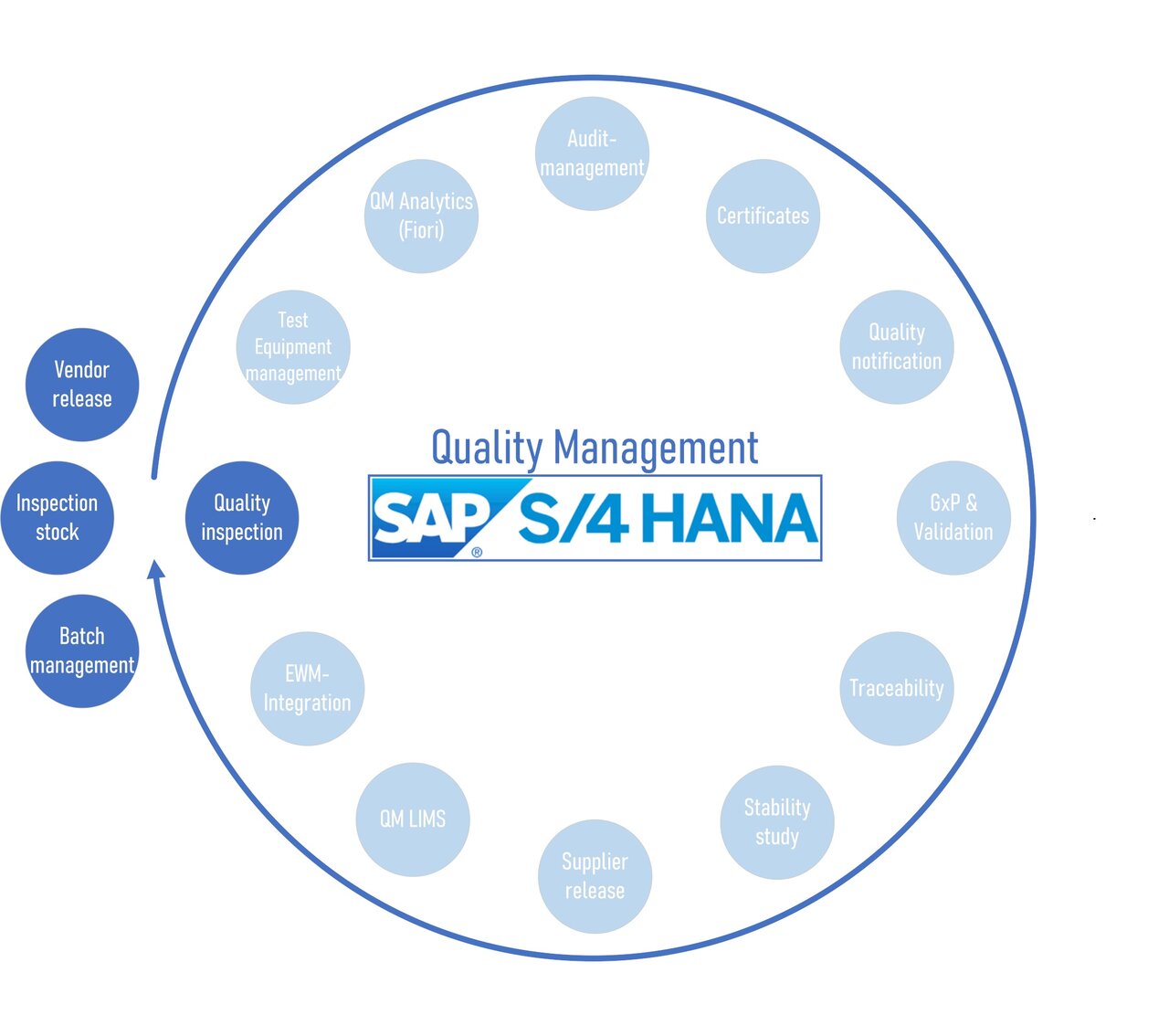
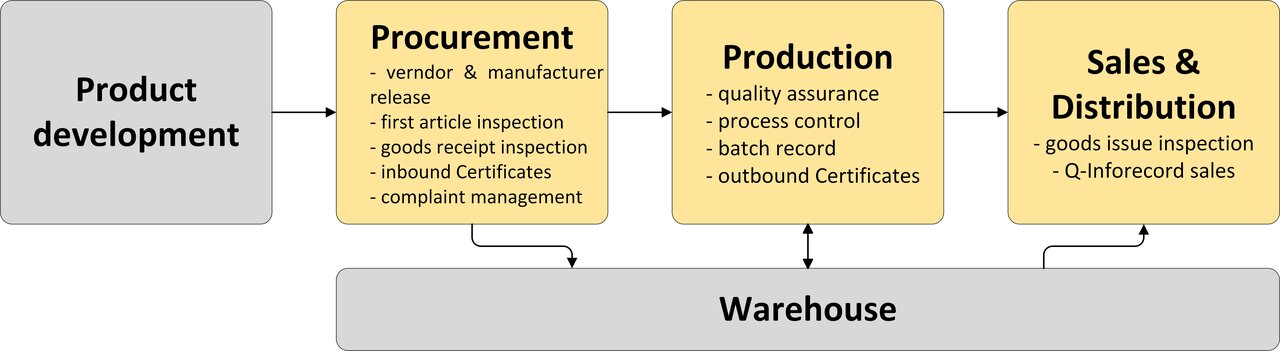
Illustration of the processes in the company
SI PRO Consulting solutions for SAP QM S4/HANA
SAP Supplier Release
Qualified and verified suppliers form the basis of a successful business. For this reason, orders may only be placed with suppliers who have successfully completed a qualification process.
For this purpose, in the SAP Supplier Portfolio Management process, authorized users can change supplier data in the SAP Supplier Portfolio and thus release potential suppliers as suppliers. The release can then be approved or rejected.
In the event of quality problems, suppliers can also be blocked again without any loss of time.
SAP Manufacturer Inspection
If the supplier of the goods is not the manufacturer, you can also record the delivery quality at manufacturer level. In this case, the manufacturer reference in SAP enables separate consideration in the various areas of SAP Quality Management, such as inspection planning, vendor approval, vendor evaluation, and quality notification.
SAP First Article Inspection
To ensure that a material meets your requirements when it is first received or after the production process, First Article Inspection (FAI) can be used. This is a full inspection that is performed only once.
SAP Stock Quality Inspection
To ensure that materials that have not yet been inspected do not enter the production process, they can first be automatically posted to inspection stock. Goods that have been posted to inspection stock are not part of unrestricted-use stock and are not available for stock removals.
Inspection and release of the GR with SAP
With SAP Quality Inspection, you can constantly and persistently determine whether delivered goods meet your quality requirements. In doing so, you can document a decision (e.g., okay/not okay) and use a defect catalog to assess the quality of a product.
Quality inspections in use with SAP Extended Warehouse Management (SAP EWM) can also be used in the delivery process and as an internal warehouse process.
SAP Batch Management
The link between SAP MM (procurement) and SAP QM enables a batch-managed based quality strategy. For goods receipt inspections and recurring inspections, the inspection lot receives a batch reference and allows a statement about the stability of the quality.
SAP QM Solutions

Chemical Industry
The Laboratory Information and Management System (LIMS) in SAP QM is based on processes and workflows commonly used in laboratories and thus offers numerous functions. Central components are the integrated sample management, test equipment monitoring and the possibility to create stability studies. The handling of the processes is supported by corresponding documents such as sample drawing instructions, inspection instructions, result reports and sample labelling.

Food Industry
SAP Non Conformance Management forms the basis for supplier control. The verification of compliance with these specifications is supported by the efficient and targeted inspection of quality characteristics. This guarantees control over quantity flows, raw material composition within the supplier's certification data at all times. The integrated message management helps to act across departments and assists in the fast handling of supplier complaints.

GxP for Pharma
The manufacture of pharmaceuticals is subject to clearly defined legal requirements. SAP meets these compliance requirements with a range of functionalities that enable GxP-compliant implementation of QM processes, as well as supporting the necessary system validation. These functions guarantee continuous and seamless documentation along the entire product value chain. This enables complete change control through the integrated audit trail as well as batch tracking and tracing for all goods movements within the supply chain of integrated SAP logistics modules.
Customers
Why you should choose
SI PRO

SI PRO has been advising on SAP QM solutions since the earliest versions were released, and offers the most comprehensive level of best practice know-how.

Our consultants are familiar with specific issues, business processes, the latest SAP solutions, technologies, and appropriate procedures.

We can support you throughout your project’s entire life cycle, from analyzing your requirements to project planning, implementation, and maintenance.
Business Contact Managing Director
Lutz Dannemann
Tel. +4962130982615
E-Mail

Business Contact Partner
Daniel Mielach
Phone +498982939455
E-Mail








